How to jumpstart innovation in R&D? Biotech must solve the instrument connectivity challenge
How to jumpstart innovation in R&D? Download your copy today.
Reaching digital maturity with a connected lab
In the 1960s, biologists were quick to embrace the new technology of the era: computers and the internet. That’s what set the foundation for bioinformatics — computational methods started to be applied to protein sequence analysis like de novo sequence assembly, biological sequence databases, and substitution models.
But despite the initial gravitation toward new technology, the R&D industry has failed to keep pace, even as life sciences data generation has exploded. The internet of things (IoT) has begun to permeate research equipment, but many instruments critical to R&D — from PCR machines to gel imaging or microscopes — are still operated manually, without connectivity with other digital tools.

In particular, life sciences and biopharma are lagging behind in incorporating data connectivity into their workstreams. In a Harvard Business Review analysis measuring digital maturity by industry, chemicals and pharmaceuticals, and healthcare ranked low. In comparison to other industries, including oil and gas, professional services, and finance and insurance, life sciences and biopharma came up short in the categories of assets, usage, and labor. There are many reasons for biology’s slowing adoption of technology and data connectivity in the lab, but the proliferation of disparate, disconnected lab instruments and scientific software systems is central to the challenge.
Consider the suite of instruments a modern lab relies on today. In any given lab, analytics instruments, cell culture systems, desktop instruments, robotics and automation systems, and next gen sequencers are amongst the many. With each of these housing a large amount of raw data, scientists are sitting on a landmine of potential innovation.
While improving lab instrument connectivity won’t solve all of the problems associated with digital maturity, it is a critical step in the right direction. By collecting and standardizing the raw data being produced across connected lab instruments, we can get maximum value from it.
In Benchling’s 2023 State of Tech in Biotech Report, we surveyed over 300 biopharma and R&D IT professionals and found that biopharma sees value and is actively investing in it.
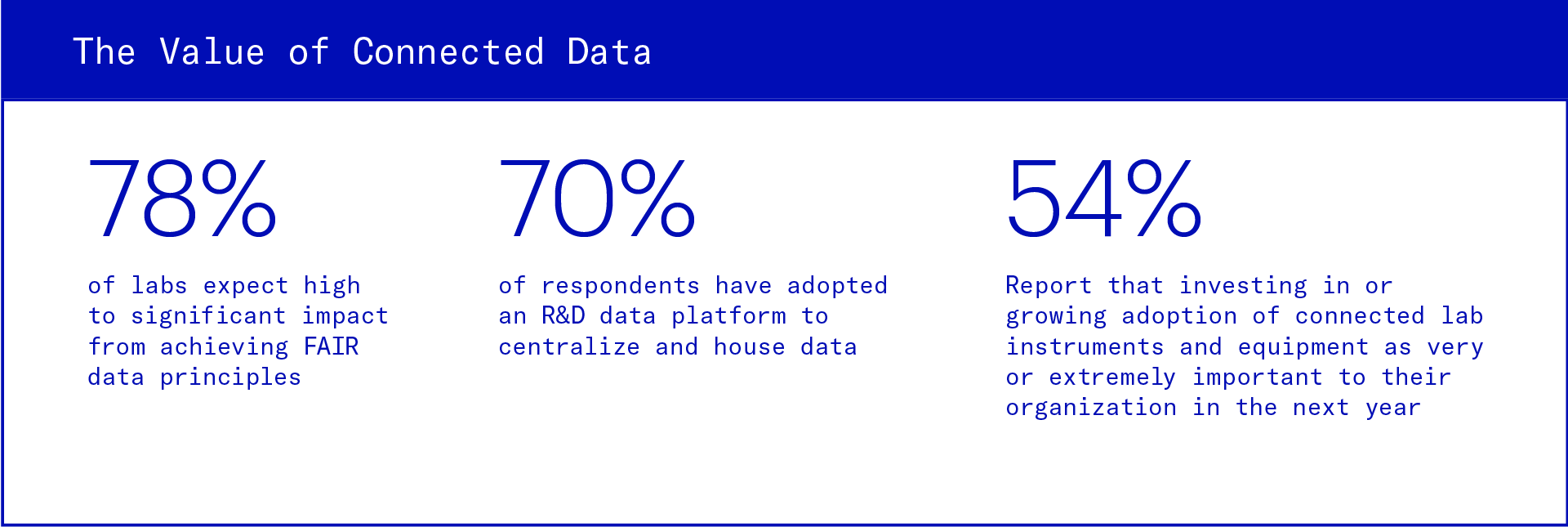
Siloed data leads to stalled decisions
In the 2023 State of Tech in Biotech Report we found that there are several compounding, universal challenges that teams are working to overcome.
Disconnected lab instruments
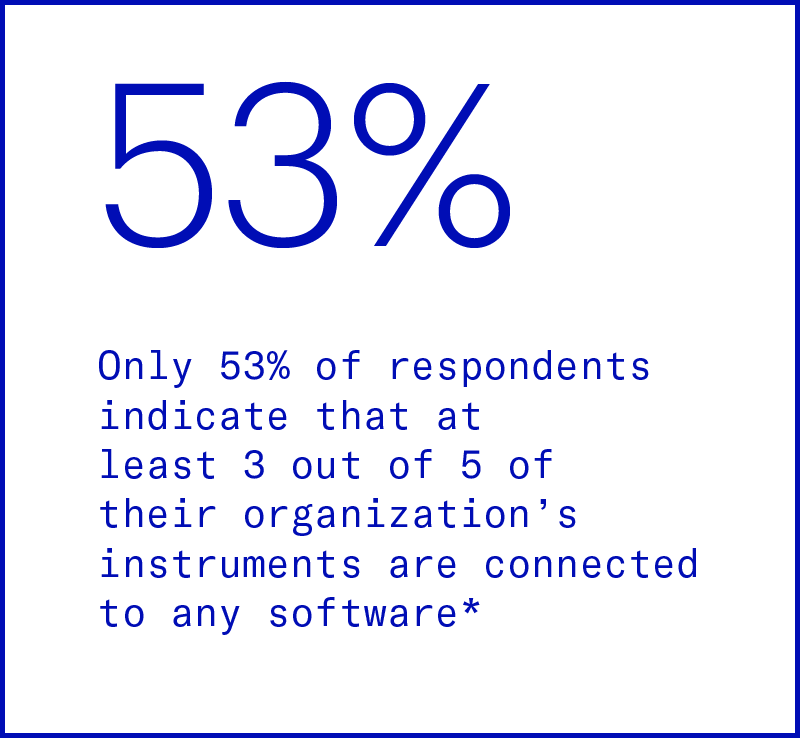
Lab operations remain largely manual and inefficient, causing a drag on scientist productivity. Manual operations also introduce more error, putting data quality, loss, and re-runs at high risk. Without a centralized, standardized method for managing data, inefficiencies extend downstream, resulting in adverse implications for analysis.
Inaccessible data
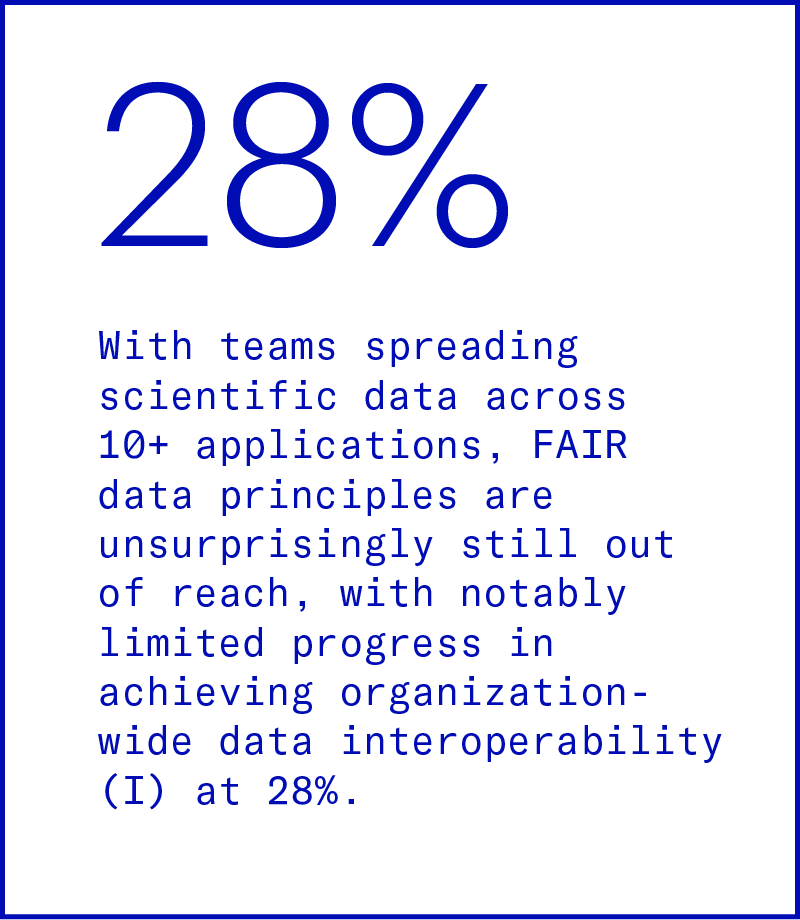
Relying on lab results without experimental context sets a high bar for scientists to understand, query, and leverage data. Because data sources are siloed, any usage of data across assays, teams, and projects requires a lot of manual, error-prone work to consolidate.
Fragmented analytics
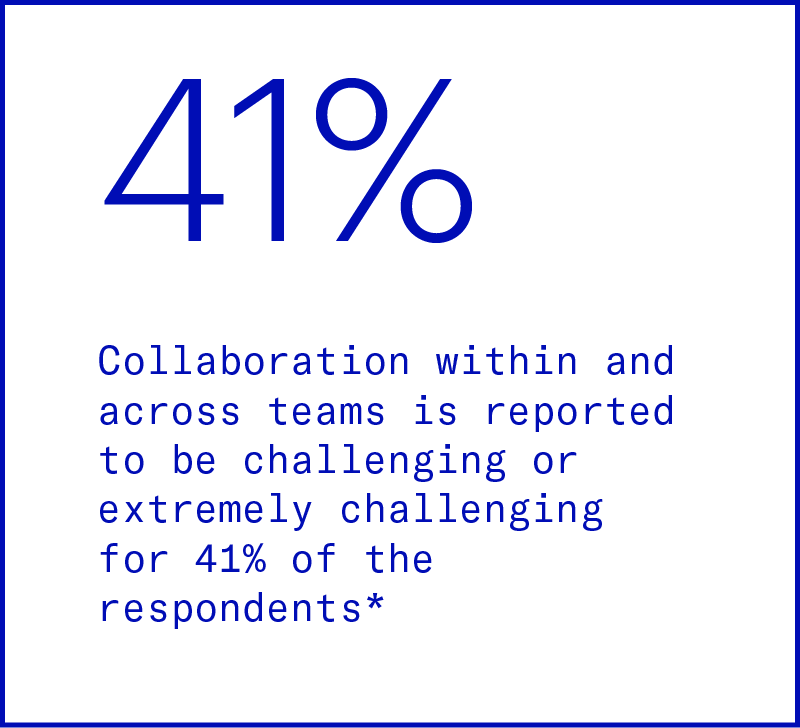
Not only are data sources siloed — data pipelines are disconnected from analysis tools as well. This means scientists and operational managers are spending more time on creating analysis than they are on making decisions.
Powering breakthroughs for over 1,300 biotechnology companies, from startups to Fortune 500s
